
Projects
The design and synthesis of next-generation carbon-based materials are pivotal to advancing material science. Our group employs a bottom-up synthetic strategy to create novel conjugated molecules, leveraging three key building blocks: metallocene, 1,8-disubstituted phenanthrene, and fluorenone. These molecules exhibit unique optoelectronic properties and hold significant promise for applications in material science.

(1) Metallocene-based Conjugated Macrocycles
Metallocene-conjugated polymers are a class of functional materials with exceptional properties and broad application prospects. However, challenges such as limited solubility and difficulties in modification have hindered their widespread use. Our group has pioneered the design of side-chain conjugated macrocycles—that significantly enhance solubility while preserving their conjugated properties. This innovative approach provides new insights into structure-activity relationships and opens avenues for the development of advanced materials.
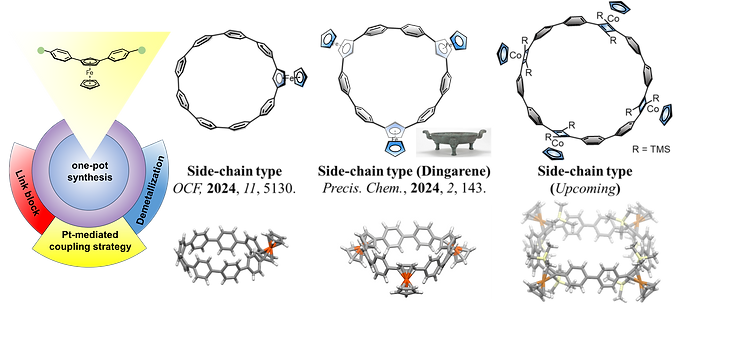
Figure 1: Metallocene-based Conjugated Macrocycles
(2) Phenanthrene-based Ladder-type Conjugated Molecules
The efficient synthesis and precise structural control of ladder-type conjugated molecules are critical for the development of high-performance carbon materials. Our group utilizes 1,8-diarylphenanthrene as a key linking unit, integrating functional building blocks such as pyrrole and thiophene, along with biphenyl end-capping units. Employing a "shotgun" synthesis strategy, we have efficiently constructed multiple series of ladder-type conjugated molecules with varying lengths. These molecules have been thoroughly characterized to elucidate their structure-activity relationships, paving the way for tailored material design.

Figure 2: Phenanthrene-based Ladder-type Conjugated Molecules
(3) 4,5-π-Extended Fluorenone
π-Extended fluorenones are a class of molecules with immense potential due to their unique electronic structures and exceptional optoelectronic properties. Our group has extended the π-conjugation of fluorenone by introducing 6/7/8-membered rings at the 4,5-positions, resulting in broad light absorption ranges. Additionally, the carbonyl group serves as a versatile functional handle, enabling the synthesis of novel spirocyclic compounds and stable radicals. This work provides new design principles for advanced carbon-based materials.
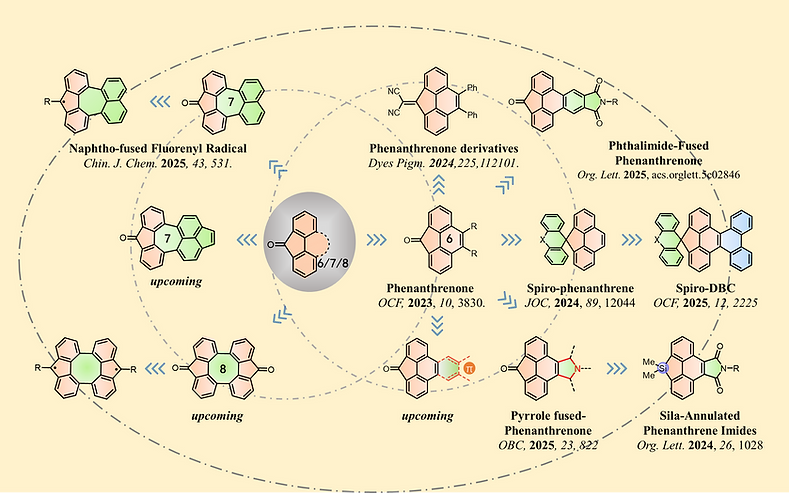
Figure 3: 4,5-π-Extended Fluorenone